Establishes the baseline QMS structure required for compliance with global medical device regulations (e.g. EU MDR/IVDR).
DIN EN ISO 13485
Medical devices – Quality management systems – Requirements for regulatory purposes
383
0
This International Standard specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Such organizations can be involved
in one or more stages of the life-cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device and design and development or provision of associated activities (e.g. technical support). This International Standard can also be used by suppliers
or external parties that provide product, including quality management system-related services to such organizations.
Requirements of this International Standard are applicable to organizations regardless of their size and regardless of their type except where explicitly stated. Wherever requirements are specified as applying to medical devices, the requirements apply equally to associated services as supplied by the
organization.
The processes required by this International Standard that are applicable to the organization, but are not performed by the organization, are the responsibility of the organization and are accounted for in the organization’s quality management system by monitoring, maintaining, and controlling the
processes.
If applicable regulatory requirements permit exclusions of design and development controls, this can be used as a justification for their exclusion from the quality management system. These regulatory requirements can provide alternative approaches that are to be addressed in the quality management
system. It is the responsibility of the organization to ensure that claims of conformity to this International Standard reflect any exclusion of design and development controls.
If any requirement in Clauses 6, 7 or 8 of this International Standard is not applicable due to the activities undertaken by the organization or the nature of the medical device for which the quality management system is applied, the organization does not need to include such a requirement in its
quality management system. For any clause that is determined to be not applicable, the organization records the justification as described in 4.2.2
Value of Implementation
Implementing ISO 13485:2021 is essential for organizations using additive manufacturing (AM) to produce medical devices or components. It is the globally recognized quality management system (QMS) standard for regulatory compliance in the medical device sector and is often a baseline requirement for market entry. For AM-produced medical products, ISO 13485 ensures conformity with stringent regulatory and customer requirements by providing a framework for risk management, process control, documentation, and traceability.
While ISO 13485 is not specific to AM, it is indispensable for establishing compliance in the medical field. To address the unique characteristics and challenges of AM technologies, it should be implemented alongside ISO/ASTM 52920, which defines AM-specific quality system requirements. Together, these standards form a comprehensive framework for ensuring consistent, safe, and effective production of AM-based medical devices within a regulated environment.
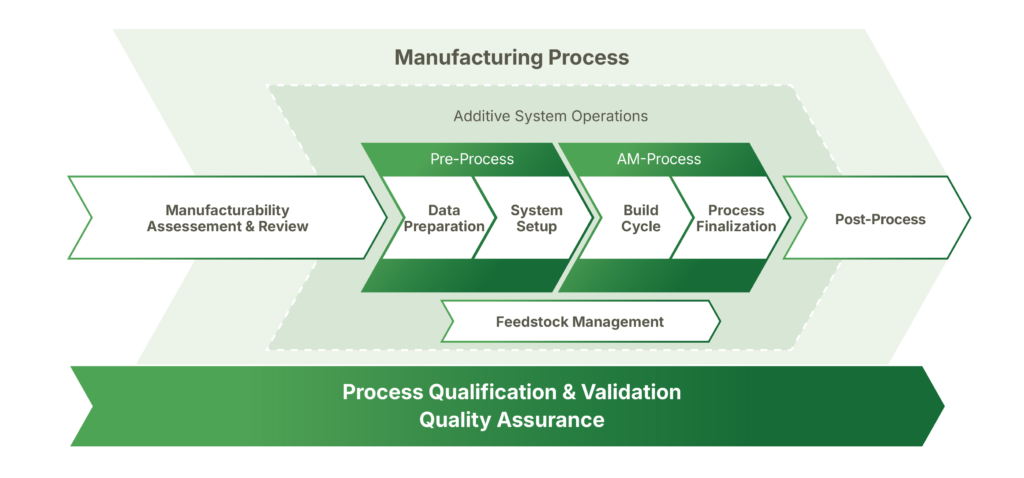
Correlation of the operational areas of AM
Standard Setter Role Overview
User Benefits

Supply Chain Manager

Quality/Production Engineer

Process/Operation Engineer
Supplier related with DIN EN ISO 13485

